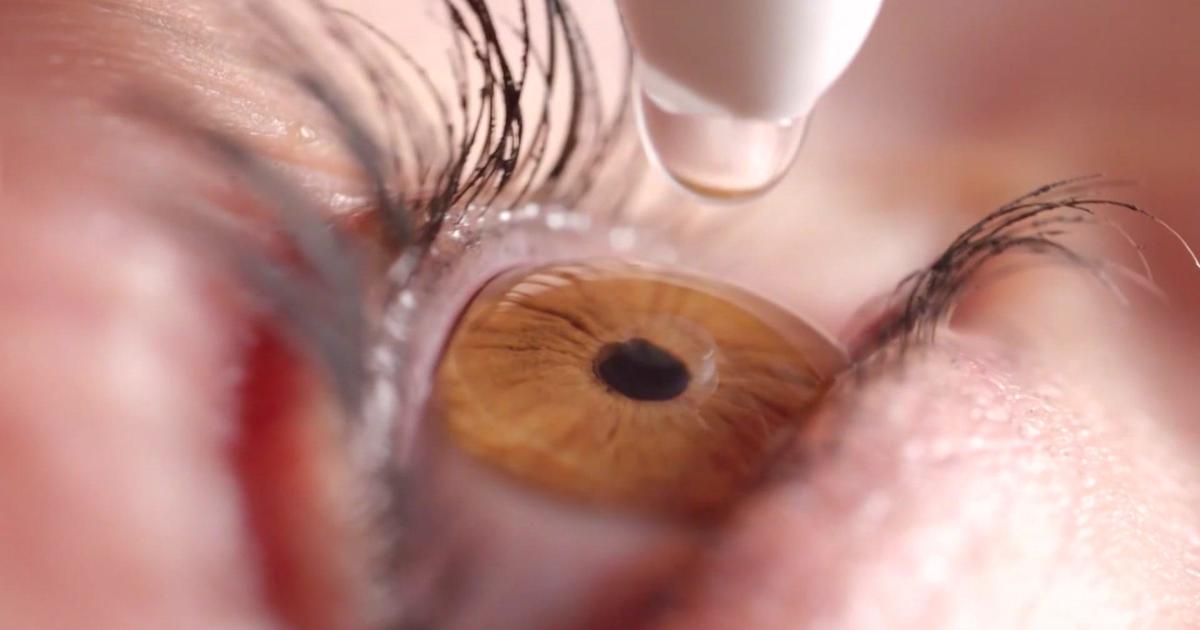
Yes, the FDA warns against using eye drops from multiple brands due to the risk of infection.
According to the Food and Drug Administration, twenty-seven different types of over-the-counter eye drops carry a risk of infection that can lead to blindness. Many people use eye drops to treat certain eye problems, such as allergies, dryness, irritation, and redness. Recent online search trends show that some people are wondering whether the Food and …