Find out what clicks on FoxBusiness.com
The Food and Drug Administration (FDA) is warning consumers about more than two dozen eye drops that the agency says could cause eye infections and potentially lead to vision loss.
Despite recent warnings, experts say consumers shouldn’t avoid all eye drop products, especially their prescription medications. Instead, consumers should avoid any products included in the FDA’s list of products that could pose a potential risk of eye infections and, in some cases, lead to blindness.
Prescription products contain preservatives and, therefore, are protected against bacteria growth, Dr. Abha Amin, an ophthalmologist and cornea specialist at Westchester Medical Center in Valhalla, New York, told FOX Business. The products on the FDA list are over-the-counter eye drops intended to treat dry or irritated eyes.
EYE EYE SOLD AT CVS, RITE AID, TARGET COULD CAUSE EYE INFECTIONS AND VISION LOSS, FDA SAYS
To date, the FDA is asking consumers to avoid or throw away 27 products marketed under brands including CVS Health, Rite Aid and Target’s Up & Up after discovering there were “unsanitary conditions” in the company’s facilities. maker.
According to the FDA, positive bacterial test results were also obtained during environmental sampling in critical drug production areas of the facility.
Although the FDA did not disclose which bacteria was found, Amin said it appears to be more aggressive given that it was able to survive the manufacturing process.
A person uses a dropper to put artificial tears in their eyes. (iStock/iStock)
If artificial tears are sterile, they should never be able to enter the eyes. They are made to sit on the surface of the eye, but if they are contained in bacteria, they will be able to enter the eye socket and then the cornea, causing problems such as conjunctivitis or a corneal ulcer, said Amin.
“If it’s not treated, it becomes a loss-of-an-eye situation. The idea being that if you have a problem and you see someone, we can try to prevent that from going too far,” she said. “We can try to stop the bacteria.”
The FDA warned that if consumers begin to develop symptoms of an eye infection after using any of the products, they should seek medical attention immediately. Symptoms include irritated or red eyesworsening pain in or around the eyes (even after contact lenses are removed), sensitivity to light, sudden blurred vision, or unusually watery eyes or discharge, according to the Centers for Disease Control and Prevention.
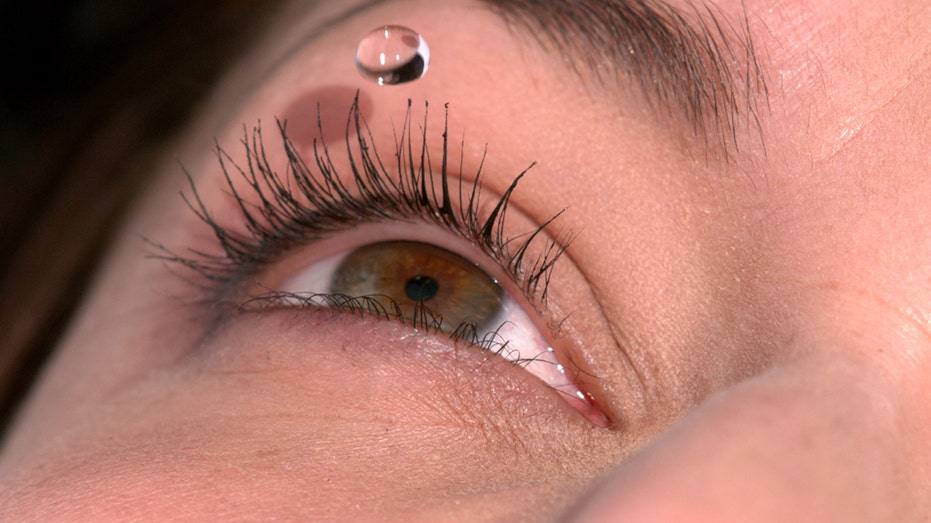
A person uses eye drops in the eye. (White Fox/AGF/Universal Images Group via Getty Images / Getty Images)
EYE DROP RECALL: FDA FINDS STERILIZATION PROBLEMS AT GLOBAL HEALTH PHARMA FACILITY
Regardless of whether they experience symptoms, consumers should discard the product immediately, the FDA and Amin said.
She also warned that the longer the bottle sits out, the more bacteria will multiply.
“Instead of 10 bacteria, you put 10,000 bacteria. Now how can the body defend itself against a concentrated bacterial load?” she says.
How to know if you need to get rid of your eye drops:
If someone has contaminated the bottle themselves or it is discolored, throw it away, Amin said.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
It is not necessary to store the bottle in the refrigerator, but it should be kept in the shade or a cooler place. It should not be stored in the sun or in a hot place.
#Eye #Drop #Products #Consumers #FDA #Warning
Image Source : www.foxbusiness.com

